Therapeutics
ACS, IMT-358, and the potential for improving cardiac outcomes
ACS, IMT-358, and the potential for improving cardiac outcomes
Acute coronary syndrome (ACS), either a heart attack or angina pectoris that can rapidly lead to a heart attack, is caused by insufficient blood flow to the heart muscle due to a clot (“thrombosis”) in a coronary artery. This causes heart muscle to be starved of oxygen and nutrients which causes cardiac instability that can lead to immediate cardiac arrest and causes permanent damage (infarction).
ACS manifests as chest pain or discomfort, shortness of breath, dizziness, nausea, sweating, and discomfort in left or both arms, the jaw, neck, back or stomach. At that point, immediate medical attention is needed.
Thrombolytic (“clot busting”) therapy, antiplatelet drugs, and the direct opening of the coronary artery via percutaneous coronary intervention (PCI: angioplasty) and coronary bypass surgery. All these procedures are performed after arrival at the hospital, by when the heart has already suffered significant irreversible damage due to heart muscle cell death.1
IMT-358 is an investigational drug under development by IMMEDIATE Therapeutics to provide metabolic protection, potentially to to minimize cardiac damage and instability during ACS.
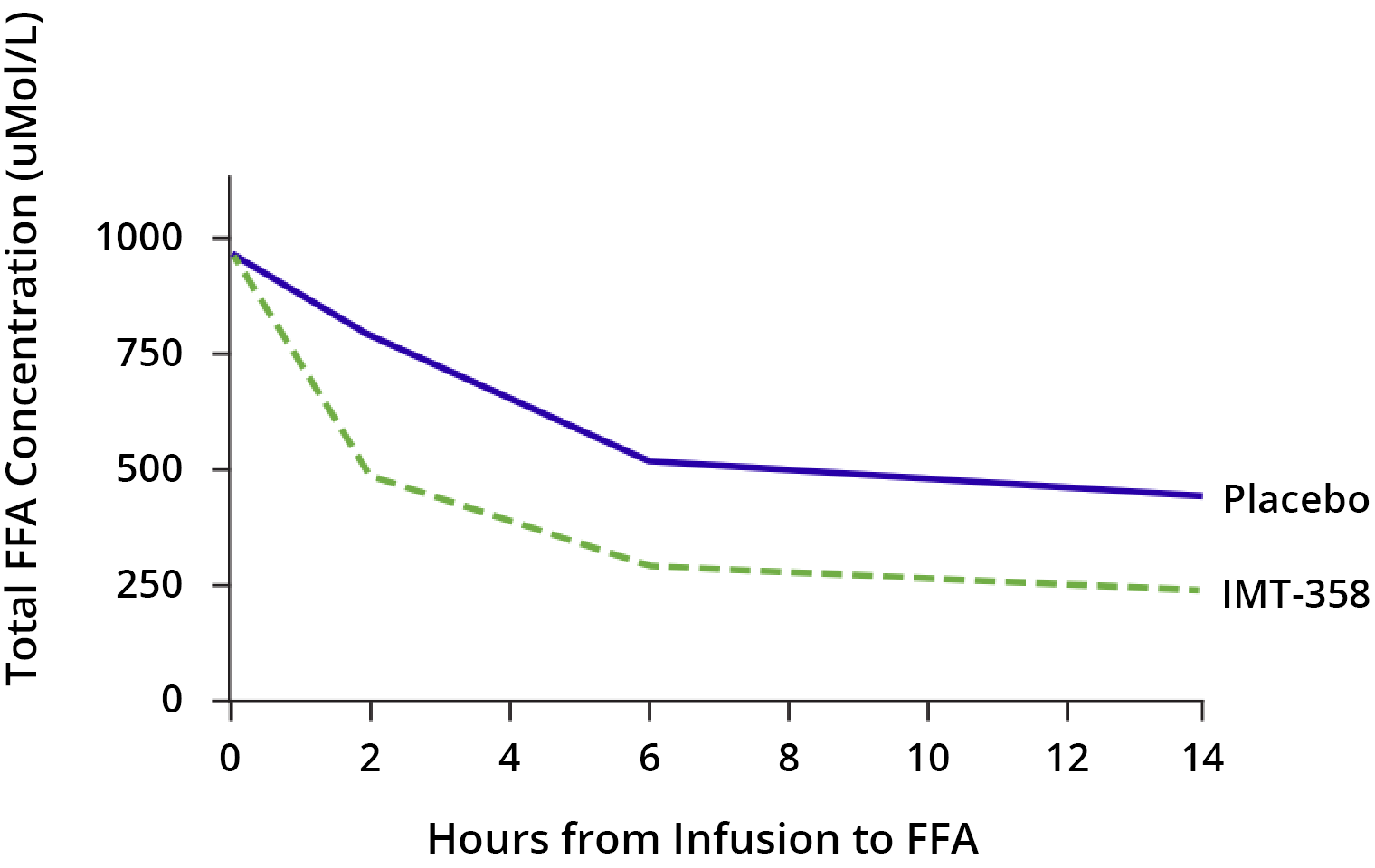
Although the mechanism of action of IMT-358 has yet to be definitively established, during ACS, the components of IMT-358 provide glucose to the heart muscle tissue. This glucose may help sustain metabolism and prevent arrhythmias, and the insulin may act as an anti-apoptotic (countering cell death) and anti-inflammatory agent. In preclinical studies2 and clinical trials3 to date, IMT-358 has been observed to reduce the buildup of free fatty acids (FFAs) that may contribute to arrhythmias and cardiac arrest.3 (68 patients in IMT 358 and 69 in placebo)
In the original IMMEDIATE trial (IMMEDIATE-1), IMT-358 was administered as soon as possible after the onset of symptoms of ACS, in the ambulance following a 9-1-1 call, or at the hospital emergency department. IMMEDIATE-1 showed that time-to-treatment after the onset of ACS symptoms was a critical factor.4 The timing of administration of IMT-358 will be further evaluated in the upcoming Phase 3 trial.
Patients in the IMMEDIATE-1 trial who were treated with IMT-358 showed 80% smaller infarcts (dead heart muscle), and far fewer large and very large infarcts, with far more of the infarcts being small or undetectable. In addition, IMT-358 also reduced cardiac arrest or mortality by 50% among all patients with ACS symptoms.4
Patients, healthcare providers, and payers could benefit from IMT-358 therapy, if and when approved by the FDA.
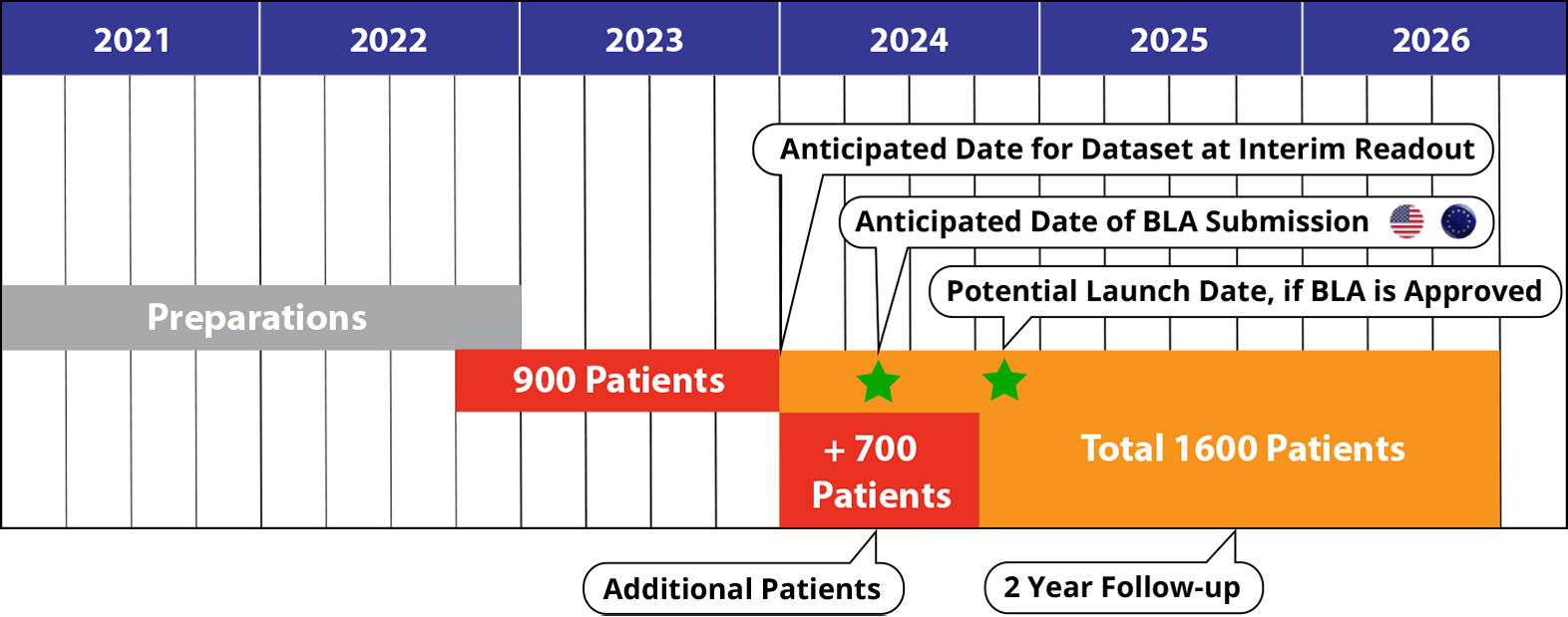
IMMEDIATE Therapeutics is preparing to for a Phase 3 trial (IMMEDIATE-2) of IMT-358. We are aiming to enroll 1600 patients and expect to initiate the trial in Q4 2022.
We are planning to explore the use of IMT-358 for patients with high risk for complications from major surgery, those who are older and/or have conditions such as diabetes, cardiac or lung disease, or other factors that make major surgery riskier. We also intend to investigate if IMT-358 will have any impact for other acute conditions beyond ACS and the risk of major surgery for e.g., in Sepsis and Stroke.